Scientists know a good deal about Huntington’s disease, an inherited neurodegenerative disorder that slowly robs patients of their physical and mental health.
They know, for example, that it is caused by mutations to a particular gene; that these mutations involve the excessive repetition of tiny stretches of DNA bases known as CAG repeats; and that these repeats corrupt the proteins that the gene produces, ultimately causing neurons in certain parts of the brain to die.
But they have not yet learned enough about the molecular mechanisms underlying this process to have developed drugs that can stop or reverse the disease.
Hence the excitement when researchers in Nathaniel Heintz’s Laboratory of Molecular Biology recently teased out some hitherto unknown nuances of those mechanisms, providing potential targets for therapeutic interventions.
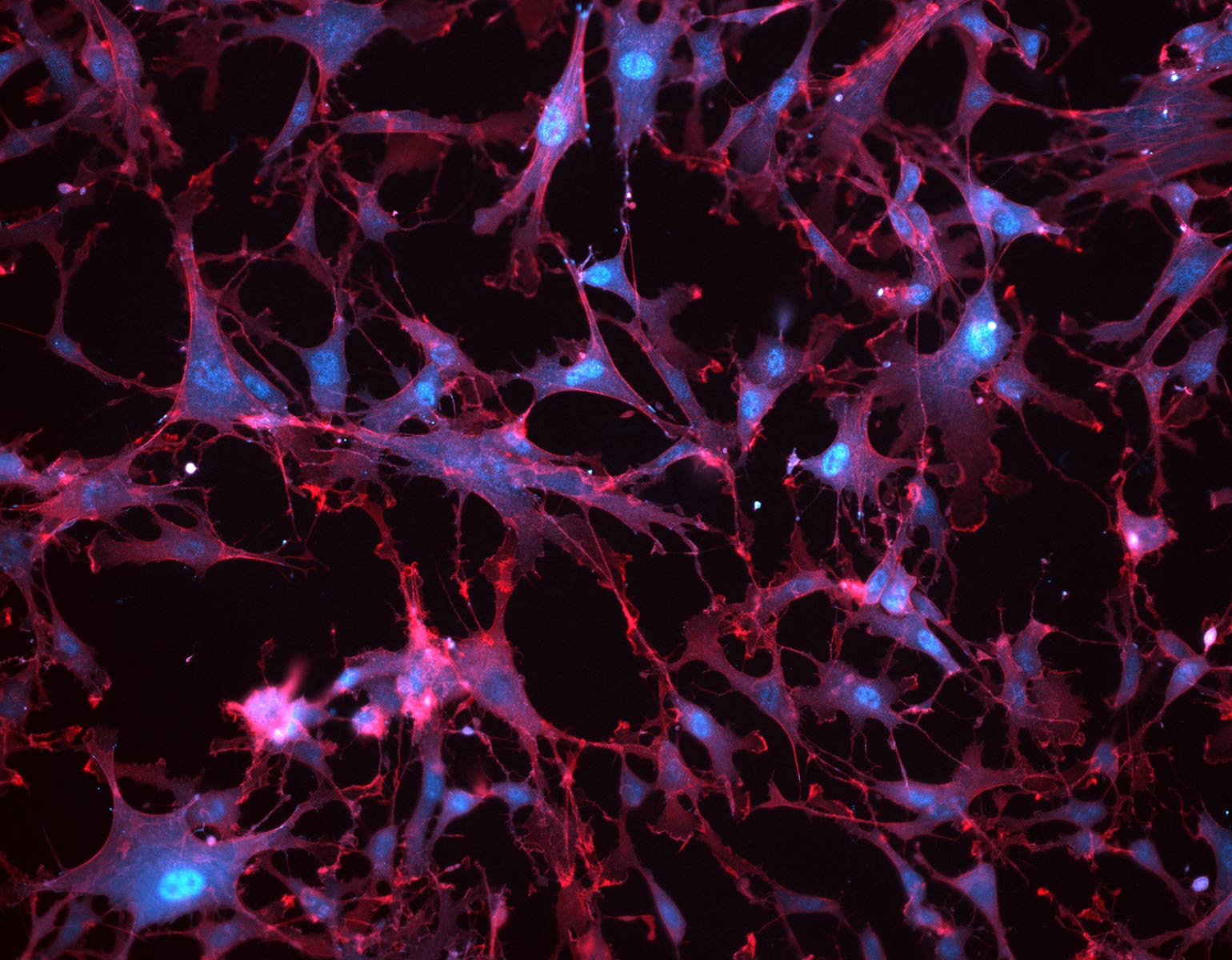
Using cutting-edge molecular profiling techniques, the scientists discovered that CAG repeats are unstable—and therefore likely to produce more toxic proteins—in only certain types of brain cells. They also found that other cell types proved surprisingly resilient to the repeats.
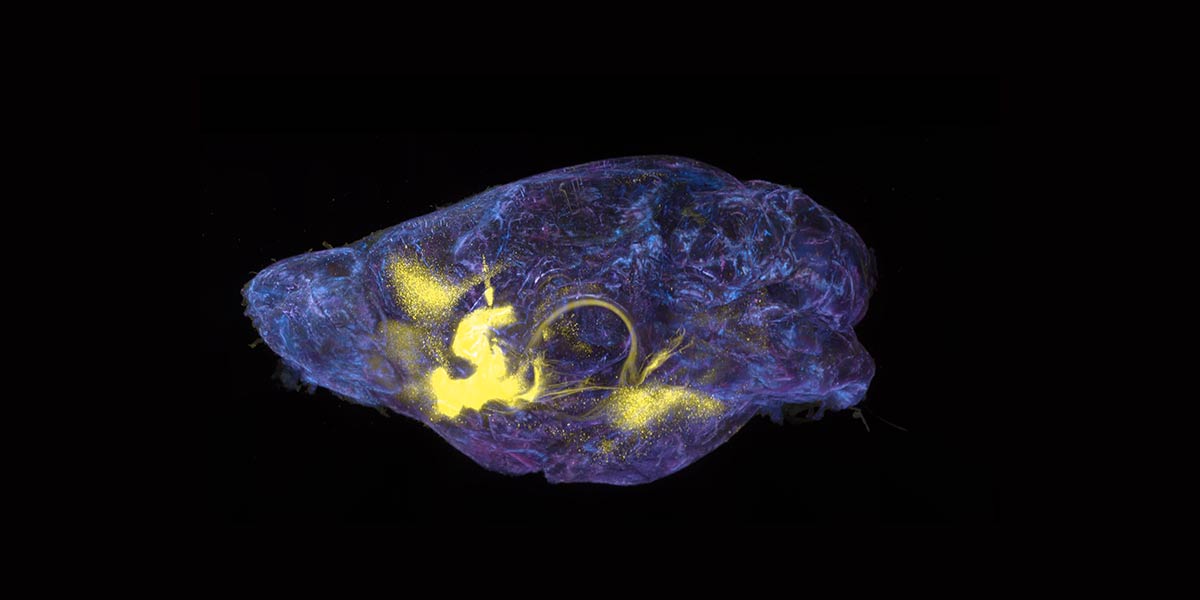
In separate but complementary studies from Heintz’s lab, Kert Mätlik, a research associate, and Christina Pressl, an instructor in clinical investigation, examined the brain cells of people who had died from Huntington’s. The scientists focused on two brain regions that are profoundly affected by the disease: the striatum and the cortex.
In the striatum, Mätlik found that CAG repeats were particularly unstable in medium spiny neurons, the cells most likely to be lost during the progression of Huntington’s. Moreover, in their nuclei these neurons contained high levels of a protein complex called MutSβ that is known to promote expansion of CAG repeats in experimental models.
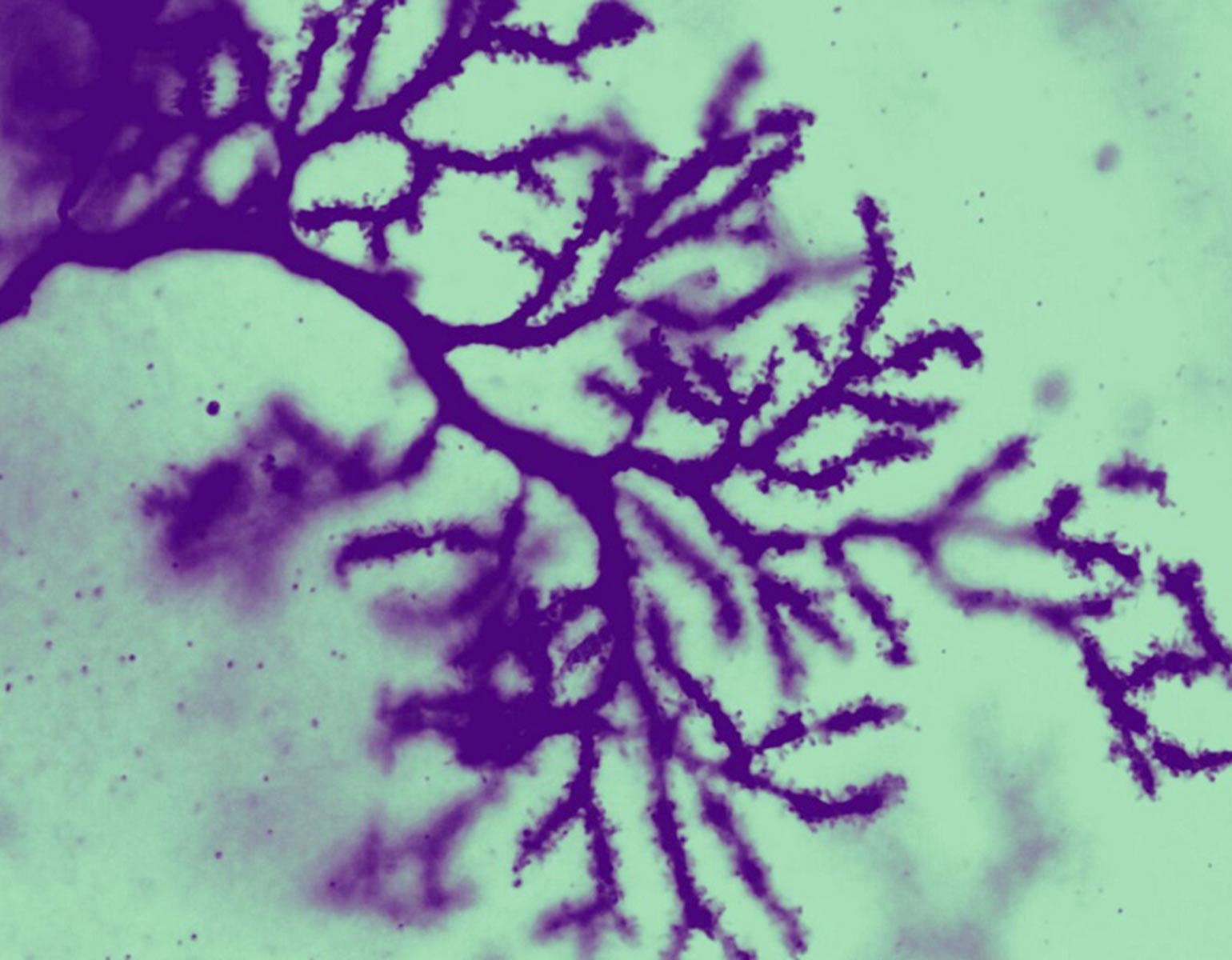
Meanwhile, Pressl discovered that although different types of pyramidal neurons found in the deep layers of the cortex had very long CAG repeats, only one population was more likely to die. The discovery “puts another cell type on the map for increased vulnerability to the disease,” she says.
Her study also provided evidence that the susceptible neurons in the cortex project slender fibers called axons into the striatum, where Mätlik’s medium spiny neurons reside; communication between the two brain regions is already known to falter in Huntington’s. As such, the team’s combined findings suggest that the vulnerable cells in both areas may be connected. “When it comes to Huntington’s, the entire neural network breaks down at some point,” Pressl says.
Heintz, the James and Marilyn Simons Professor, hopes to build on these studies to answer more questions about the disease.
“Is there a specific length of repeats at which the cells become dysfunctional? At what CAG repeat length do the cells die, and does it differ depending on the cell type?” he asks.
“We need to understand these things in order to develop new treatments for this devastating disease.”