Feature
Maybe the virus isn’t the problem
By Eva KieslerThree years into the pandemic, the scientific response has delivered beyond expectations. We have effective vaccines against COVID, we can treat it with antivirals, we have practical strategies for avoiding it. Yet how the virus impacts people’s health can still seem mysterious. Why is it, for example, that some end up on ventilators and others get by with a scratchy throat or no symptoms at all? Why do some recover fully within weeks while others struggle for months or years with long-COVID symptoms? And most puzzling of all, why do some individuals appear to have dodged the virus this whole time?
Answers are now emerging from hundreds of scientists around the globe who joined forces when the first wave got underway. As the world fixated on the new virus, these experts looked beyond that spiky orb to focus on the human side of the equation—specifically, on variations in our genome that determine what will happen when a person comes in contact with SARS-CoV-2. Their discoveries are changing the way we think about infection, not only in the context of COVID, but for any illness set in motion by a microbe.
In the winter of 2020, right after news emerged about a new coronavirus outbreak in Wuhan, China, Jean-Laurent Casanova, an immunologist with laboratories in New York and Paris, sat down to write the most important email of his career. He would send it to nearly a thousand people. “I wrote everyone I had ever worked or corresponded with,” he says. “Absolutely everyone.”
He is an avid correspondent, with friends and collaborators around the world. But this email was different and would quickly move the needle on research into COVID.
“There’s been a stunning inter-individual variability between patients,” he typed, referring to the erratic way in which the disease was rippling through communities. It was already evident that elderly people and those with underlying conditions were at risk for the worst outcomes; but something strange was happening among those who were young and previously healthy. While most had manageable symptoms or no symptoms at all, a seemingly arbitrary group was contracting severe or fatal forms of the disease.
There was no way to predict who might live and who might die from an infection, and the uncertainty was setting off a secondary pandemic of anxiety and fear. But Casanova, the Levy Family Professor at Rockefeller, had a strong suspicion where the reasons for the discrepancies might lie.
“We have extensive knowledge about pathogens, but we still don’t understand why they make some people critically sick.”
“Please join our effort!” he continued in bold, red font. “We need whole blood, fresh or frozen, or genomic DNA from patients.”
Hundreds of his colleagues responded, and thus was born the COVID Human Genetic Effort (CHGE), an international consortium that Casanova co-leads with Helen Su, chief of the Human Immunological Diseases Section of the National Institute of Allergy and Infectious Diseases. Now in its third year, CHGE brings together more than 400 scientists and close to 40 DNA sequencing labs across six continents, and it’s well on its way to answering the pandemic’s most perplexing questions.
In sequencing and analyzing patients’ genomes, the consortium has already found flaws in immunity that might explain up to 20 percent of critical cases, an achievement stemming from discoveries made in just a handful of people. It’s the kind of work that simply cannot be done without a global reach.
“If you’re looking for a rare mutation, there may just be one person in Hong Kong and another person in Buenos Aires who have it,” Casanova explains. “And you will never find it unless you have colleagues who can help you recruit patients from these places.”
He would know. For over 30 years, Casanova has leveraged an extensive professional network to investigate why people may respond differently to the same pathogen. Ten years ago, for example, his team found one patient in Turkey and another in Iran who carried a rare mutation that made them abnormally sick from generally harmless mycobacteria. Another time, they studied a French 2-year-old with a different mutation who nearly succumbed to the seasonal flu, which most children fight off within a week.
485Number of diseases linked to inborn mutations in immune-system genes.
So when COVID hit, Casanova and his colleagues immediately got to work collecting DNA from as many infected people as they could get their hands on. While others focused exclusively on the new coronavirus, these scientists believed the biggest mystery of the disease lay elsewhere: in the tangle of biochemical pathways that make up the human defense against it and other pathogens.
“What we’re seeing in this pandemic was also true for the 1918 flu and almost any other infectious disease,” says Isabelle Meyts, a professor in pediatrics at KU Leuven in Belgium and member of CHGE’s steering committee. “Only a fraction of infected people gets life-threatening disease. And the problem isn’t the pathogen, it is us—the problem is the genetics of the host.”
In this sense, infectious diseases are like cancer, cardiovascular disease, diabetes, and almost every other illness that scientists have scrutinized at the molecular level. They can be traced to mutations in the genes we are born with, and when such mutations are discovered, new opportunities may arise to diagnose, treat, or prevent the condition.
Thus far, however, infectious disease experts have often ignored the role of human genetics, focusing instead on the study of microbes—how they spread, how they enter host cells, how they replicate, and how to kill them. The omission dates back to the early pioneers of the field, who were more interested in germs than in genes.
This was true even for Louis Pasteur, the 19th-century chemist who discovered that microorganisms spread disease and turn grapes into wine. He was among the first to point out that infectious diseases are both infectious and inherited. Of flacherie, a silkworm plague that nearly put an end to the French Second Empire’s silk industry, Pasteur wrote that “it is not the microbe that is transmitted from parents to offspring, but the predisposition to disease.” Still he didn’t dwell long on the Mendelian aspects of infection, turning instead to more pressing matters—like convincing the world that common diseases like rabies and typhoid fever were caused by microbes rather than by “bad air” or bad morals.
As the genetic aspect fell by the wayside, Pasteur’s and other germ theorists’ work on pathogens led to spectacular new therapeutics, including vaccines and antibiotics that revolutionized public health. Yet strangely enough, these scientists had little or no knowledge of how their innovations worked or how infectious pathogens were sabotaging their hosts’ physiology in the first place.
“Medicine usually follows science,” Casanova says. “But for infectious diseases, the opposite happened: Medicine got ahead of scientific understanding very early on, and the science still hasn’t caught up. We have extensive knowledge about pathogens, but we still don’t understand why they make some people critically sick.”
Picking up where Pasteur and his contemporaries left off, Casanova and Meyts are now shifting the focus from pathogens back to their hosts—which is to say, to the patients themselves. Both are pediatricians interested in inborn errors of immunity, mutations that some people carry from birth that compromise the body’s ability to defend itself against specific pathogens (or in some cases, against multiple pathogens). Inborn errors represent the most clear-cut cases of genetic predisposition to infectious disease, and as such, they tend to be rare. Most of them are subtle as well: If you’re carrying an inborn error, you may not know it until you’re confronted with a germ capable of exploiting that particular flaw.
In CHGE’s first initiative, Casanova, Meyts, and their colleagues recruited hundreds of patients with critical COVID—those who were ill enough to be admitted to an ICU—as well as individuals with a mild or asymptomatic infection. They then compared genomes, looking for telltale differences between the two groups. Just a couple of months into the pandemic, results emerged that were so illuminating that “COVID may already be the best understood of all infectious diseases,” Casanova says.
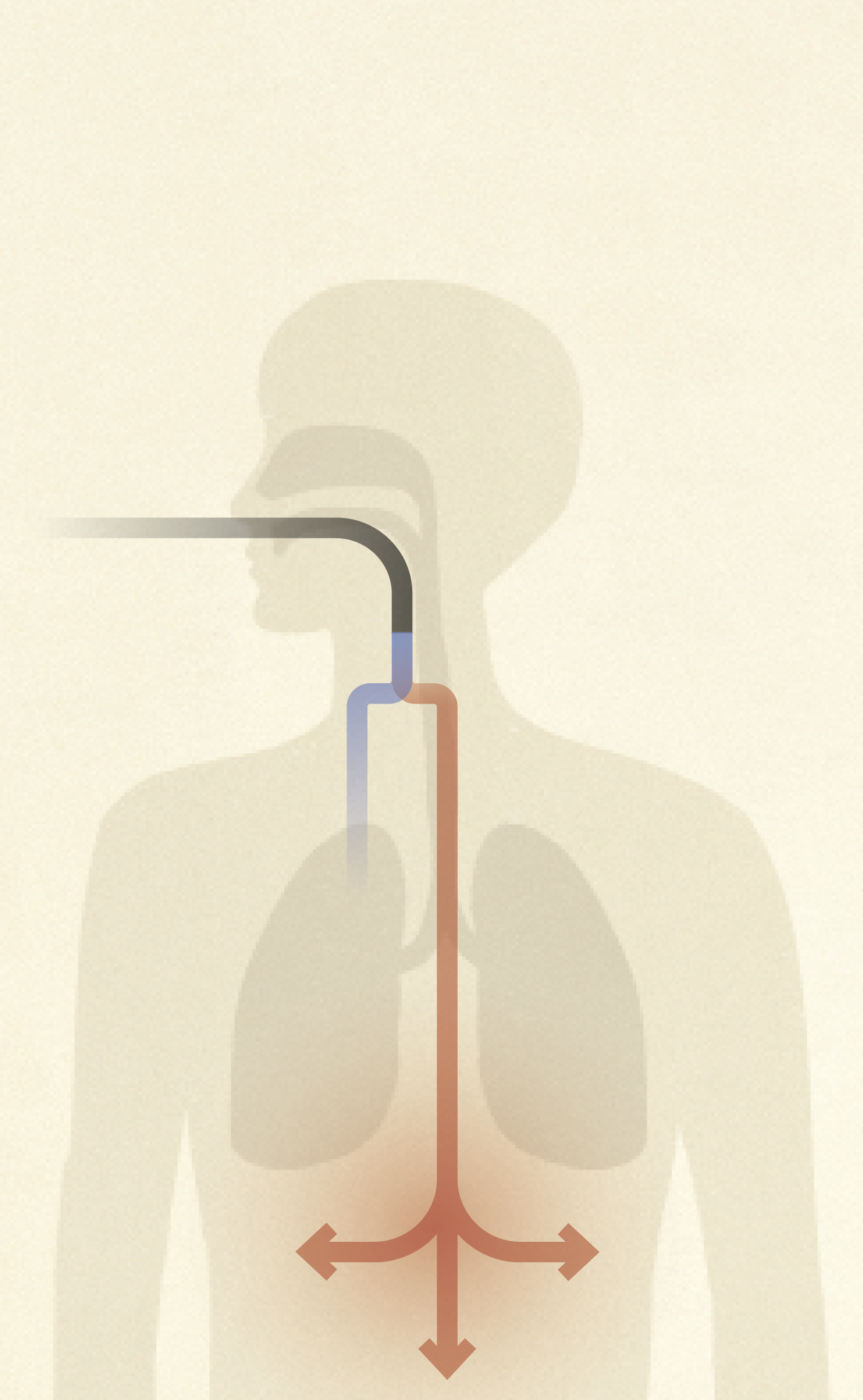
The researchers found that at least one to five percent of the critically ill carried inborn errors in gene coding for molecules that control the activity of type I interferons, a set of signaling substances released by our cells in response to some viral infections in order to prevent those viruses from replicating. Because of their warped interferon activity, these patients’ immunity lacked the ability to curtail the virus in the early stages of the disease, a glitch that may allow SARS-CoV-2 to move from the respiratory tract to the bronchi and lungs and ultimately to reach vital organs throughout the body (see “When interferon fails to interfere”).
What’s more, inborn errors turned out to be the tip of the iceberg. The team found that some critically ill people whose interferon genes were intact instead produced misguided immune molecules known as autoantibodies. In these patients, autoantibodies treat the body’s own interferon proteins as foreign entities, glomming onto them and preventing the proteins from functioning properly.
Meyts was intrigued to learn that poor COVID outcomes may arise via two different mechanisms that both sever interferon signaling. “Whether the problem is autoantibodies or interferons or the genes that control interferons themselves, we see the same phenotype,” she says. “These are beautiful findings that make a lot of sense.”
Interferon autoantibodies are much more common than interferon mutations: In analyzing pre-pandemic blood samples from more than 35,000 individuals, the researchers detected them in about one in 500 people aged 18 to 69. The team also discovered that the chance of having autoantibodies increases with age—their incidence is 1.1 percent among people aged 70 to 79, for example, and 3.4 percent among those 80 and older—which may be why many elderly people die from COVID.
Taken together, the inborn errors and autoantibodies discovered thus far account for approximately one in five severe cases. But the researchers believe interferon deficit may be driving even more ICU admissions and deaths through mechanisms yet to be discovered. Moreover, the team recently detected interferon autoantibodies in people with severe influenza, and they suspect that the same mechanism may be responsible for bad outcomes in a range of other viral diseases as well.
“We will definitely know more soon,” Meyts says.
In the meantime, other CHGE scientists are looking at the flip side of severe COVID: the possibility that some people carry gene variants that protect them from the disease. It’s an intriguing idea but very hard to corroborate.
Anecdotally, however, the world is full people who’ve repeatedly been exposed but never been infected. “There are health care workers who worked in the ICU during the height of the pandemic whose tests kept coming back negative,” says András N. Spaan, a former postdoc in Casanova’s lab who now heads his own research team at University Medical Center Utrecht in the Netherlands. “And there are couples where one partner was sick and the other remained healthy even though the two lived together without isolating.”
Are these cases mere flukes, or could it be that some people’s genetic makeup makes them invincible to the virus—not only symptom-free but also unable to infect others? Spaan is conducting a study to find out.
The project faces distinct challenges. For one thing, there is little precedent for genetic superpowers in the context of infection. Spaan knows of just a few examples, including reported cases of people resistant to HIV, malaria, or norovirus. And recruiting volunteers can be tricky. “People typically show up in the clinic because they’re sick,” Spaan says. “Those who stay healthy are less likely to encounter specialist physicians who would enroll them in a study.”
“The genetics of inborn resistance can directly show us how to confer powerful immunity to people who don’t naturally have it.”
Nonetheless, he and Casanova have heard from thousands of people who offered to participate in the research, suspecting that they might be COVID-
resistant. “Our work has received a lot of helpful press coverage,” Spaan notes. However, he says less than 10 percent of volunteers meet the recruitment criteria, which seek to ensure that participants have in fact been exposed to the virus. “It’s one of our biggest challenges,” Spaan says. “We’re trying to distinguish between those who may be genetically resistant and those who didn’t get sick for other reasons: maybe they were vaccinated, wore a face mask, or took other precautions.”
A COVID-resistant individual would have gotten rid of the virus very quickly, either because it failed to enter host cells or because the person’s immune response immediately overpowered it.
“It’s very hard to prove that something was there at one point if it’s no longer there,” Spaan says.
The researchers are following several leads, including one emerging from another line of investigation focused on what may be the pandemic’s weirdest clinical manifestation: pernio, or “COVID toes.” Before SARS-CoV-2 came along, Ahmad Yatim, a dermatologist at Lausanne University Hospital, would occasionally see patients come in with discolored, swollen toes or fingers. These cases peaked dramatically during the first and second COVID wave, leading to the widespread assumption that pernio is a symptom of the disease. To their surprise, however, Yatim and his colleagues found the opposite. Most patients with COVID toes tested negative by PCR as well as by antibody testing, suggesting they were free of COVID and hadn’t had it in the past either.
1 in 5Estimated ratio of people aged 18 to 64 experiencing at least one medical condition due to COVID up to a year after infection. Among those 65+, the ratio narrows to 1 in 4.
“We’ve come to think that pernio is indicative of a very strong interferon response that instantly shuts down the virus,” says Yatim, who is currently conducting postdoctoral research in Casanova’s lab.
The theory makes intuitive sense: If some people get sicker than others because their interferon response is inadequate, why wouldn’t there be others capable of churning out so much interferon that the virus has no chance at replicating? The researchers believe most people fall somewhere in between these extremes, and the better your interferon response, the milder your COVID symptoms.
Still, the interferon-spectrum model remains to be verified. To that end, the team is looking for mutations that predispose to resistance. Spaan suspects such mutations might be very rare—but if they can be found, the impact would be huge.
“Inborn errors are experiments of nature,” Spaan says. They make it possible to peek under the hood of an infectious disease and start doing what centuries of pathogen-centric work has often failed to do: untangle the disease mechanisms at play in human cells and tissues. And although inborn-resistance mutations will be much harder to find than bona fide “errors”—mutations linked to poor outcomes—they tend to be more effective in pointing the way toward the development of new therapeutics.
“The genetics of resistance is a best-case scenario in which the body fights off pathogens very efficiently,” Spaan says. “It can directly show us how to confer powerful immunity to people who don’t naturally have it.”