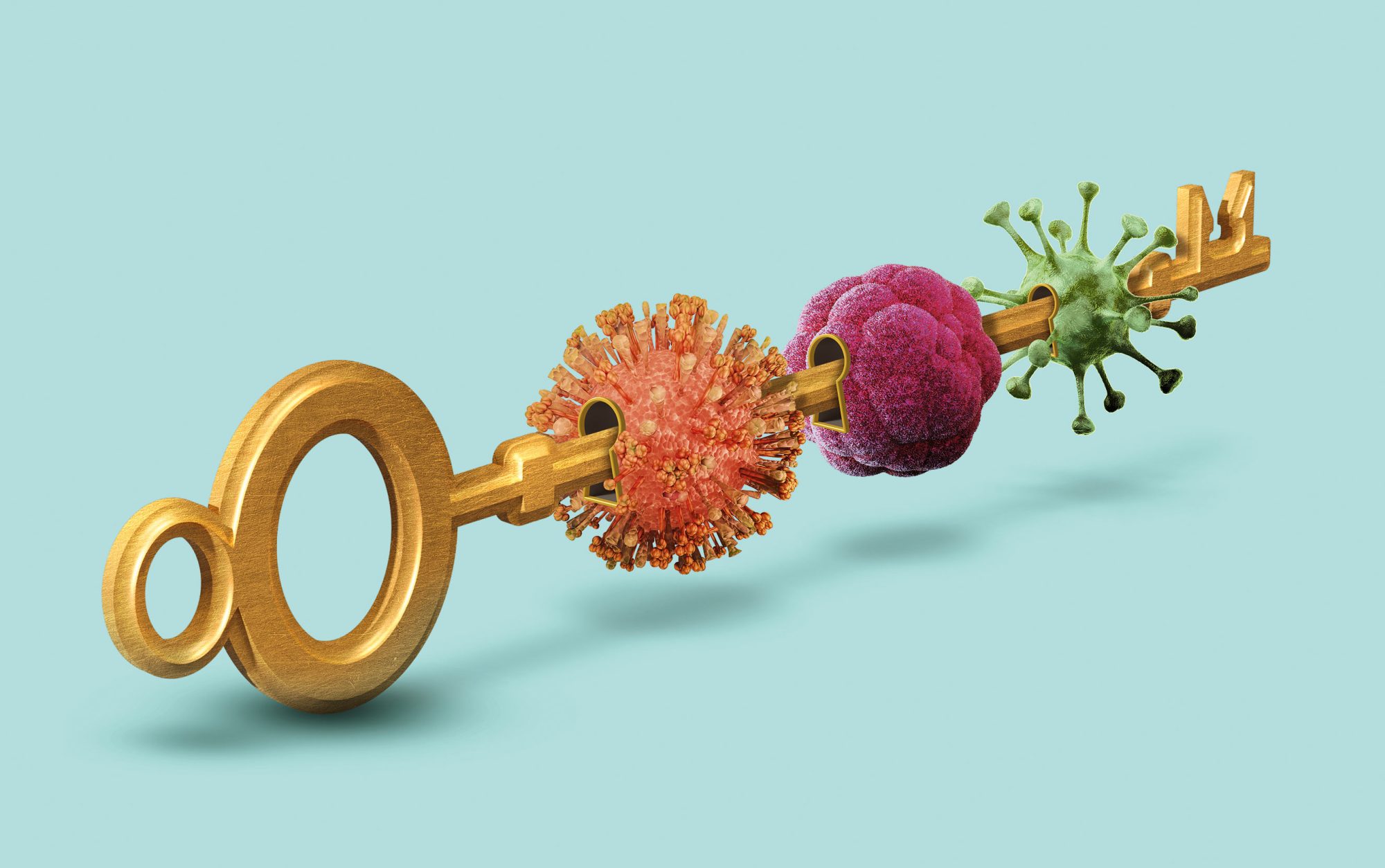
Feature
The key to better antivirals
Medicine has long been stymied by unique challenges inherent to developing antiviral drugs. But the rush to take down one virus may have unlocked new methods to knock out multiple viral foes.
By Bethany BrookshireI
n the early days of 2020, Rockefeller buzzed with its usual quiet intensity. Graduate students hunched over benches and racks of tubes. Postdocs scribbled diagrams onto glass whiteboards. And in his spartan office at the back of a sprawling lab, Thomas Tuschl was preoccupied with molecules that much of the world had barely heard of—proteins that guide RNA as it shuttles genetic material around cells.
Then everything changed. While many began sheltering at home during the COVID-19 pandemic, researchers at Rockefeller quickly began working to try to understand SARS-CoV-2, a virus that stores its genetic instructions in strands of RNA. “As an RNA scientist, I wanted to contribute,” recalls Tuschl. So his lab pivoted, searching for drugs that would shut down the virus’s genetic apparatus. At the same time, a few floors below, Rockefeller virologists Theodora Hatziioannou and Paul Bieniasz had also seized on the idea of developing antivirals, testing how SARS-CoV-2 dodged antibodies—and how to coax immune molecules to launch an attack on the virus.
Both labs had spent decades studying how viruses replicate and how the immune system fights back. “It’s hard to overstate the amount of adrenaline that was flowing,” says Bieniasz, who is the Purnell W. Choppin Professor, as well an investigator at the Howard Hughes Medical Institute.
Five years later, that adrenaline has diminished for most people; much of the world has moved past COVID-19. Pandemic-related scientific funding has expired or been withdrawn, and most labs have shifted their research back to other topics. But the virus hasn’t disappeared—in fact, it continues to mutate, even as related threats may already be evolving somewhere out of sight.
“There’s a whole collection of coronaviruses circulating in bats,” notes Hatziioannou. “Their introductions into the human population are getting more and more frequent.”
So, at Rockefeller, the work continues, and it’s been revealing insights that may have implications far beyond COVID-19. The same viral tricks used by SARS-CoV-2 are employed by viruses like dengue, MERS, and even the common cold. By investigating COVID-19’s weak points, Rockefeller’s researchers may find themselves laying the groundwork for a new generation of antivirals.
“This is not just about one pathogen,” says Nobel laureate Charles M. Rice, who leads the Stavros Niarchos Foundation Institute for Global Infectious Disease Research at Rockefeller. “If we create a drug that protects against a whole family of viruses, it could head off the next pandemic.”
It may even do more than that.
An evasive enemy
Viruses are, in a sense, the ultimate minimalists. Unlike bacteria, which have their own cellular machinery and numerous components scientists can target, viruses are little more than genetic material wrapped in protein. They don’t grow or divide. They don’t even metabolize. Instead, they invade a host cell and hijack its inner workings, transforming it into a virus-making factory. That parasitic simplicity makes them difficult to kill without harming the host in the process.
Adding to the challenges, viruses mutate incessantly. Each time a virus copies itself, it makes tiny mistakes. Most of those mutations go nowhere, but the rare ones that offer an advantage, like escaping the immune system or resisting drugs, can take over in a matter of days.
“Viruses are not smart,” Hatziioannou explains, but with how fast they seem to evolve to evade scientists’ best efforts, you’d be forgiven for thinking they are. “It’s like the virus is trying every possible move simultaneously in chess matches against you,” she says. “In most of the games it’s going to lose, and those are the variants that you’re never going to see. But in one it’s going to win, and that’s the game that counts. That’s all it needs.”
It’s no surprise, then, that while we have hundreds of antibiotics to treat bacterial infections, we have relatively few antiviral drugs, and even fewer that combat multiple viruses.
Scientists have long dreamed of something analgous: a powerful broad-spectrum antiviral that could work across many viruses, perhaps even an entire family like coronaviruses or flaviviruses. Something like the way amoxicillin works on a range of bacterial infections—simple to administer, widely effective, and, importantly, resistant to resistance.
The urgency of COVID-19 prompted labs to begin putting more resources than ever toward this goal. How could scientists stop playing catch-up and develop a drug that would protect against both currently circulating strains of COVID-19 and any future variants? Could such a drug also combat viruses beyond COVID-19?
“It’s a tall order,” Rice says. “How do you come up with an approach that gives good protection against a founding virus, and then all of the variants that it could generate?”
Stripping away a Viral Disguise
When SARS-CoV-2 burst onto the global stage, most scientists turned their attention to the spike protein—the now-infamous protrusion that allows the virus to enter human cells. But Tuschl, after years of studying RNA molecules, was looking elsewhere. He had his eye on an enzyme called NSP14, which modifies a protective cap on SARS-CoV-2’s RNA. The cap convinces human cells that the viral RNA is “self” and should be translated into proteins rather than be destroyed. Without the modified cap, the virus’s genetic material would be unmasked, exposed to the human immune system as foreign.

SARS-CoV-2 and related viruses must use NSP14 rather than human proteins to attach this genetic cap, and that made it enticing: It’s a viral enzyme that’s essential, unique to the virus, and different enough from anything in human cells that a drug could block it without harming us. “If you’re looking for a target, any viral enzyme that makes its own RNA look like it belongs to the host is a good one,” Tuschl says. “In this case, disabling the virus’s capping mechanism stops the virus from replicating and spreading out from the host cell.”
“Our work not only establishes NSP14 as a therapeutic target, it also opens the door to many more antiviral developments against pathogens that until now we’ve had only limited tools to fight.” Tuschl
So Tuschl’s lab got to work. They isolated NSP14, studied its activity, and began searching for ways to thwart it. In collaboration with Rockefeller’s Fisher Drug Discovery Resource Center, the team threw the pharmaceutical kitchen sink at it, testing more than 430,000 compounds to see if any would block the enzyme’s function.
The effort paid off. They identified a molecule that bound tightly to NSP14’s active site. Then, they made chemical tweaks to the molecule to make it even more potent. The final product, dubbed TDI-015051, acted like a molecular wrench, jamming the enzyme and preventing the virus from accessing its own RNA. When tested in cultured human cells, the drug stopped SARS-CoV-2 from multiplying. When tested in mice, it inhibited virus reproduction just as effectively as nirmatrelvir, the active ingredient in the COVID-19 antiviral Paxlovid.
“Our work not only establishes it as a therapeutic target,” says Tuschl of TDI-015051, “but it also opens the door to many more antiviral developments against pathogens that until now we’ve had only limited tools to fight.”
All coronaviruses—from SARS to some common cold strains—use similar proteins to modify their RNA caps, suggesting that this compound or its successors might someday work against all of them. Even more tantalizing: Other RNA viruses, such as dengue and Ebola, also rely on their own unique capping proteins to disguise their RNA. “This is a new entry on the list of viable viral targets,” Rice says. “This kind of work could potentially lead to a whole new class of antiviral drugs.” More pre-clinical work is needed to solidify TDI-015051 as a potential drug for humans, but Tuschl says his lab is continuing on the case, testing how to shut down other similar proteins.
Rice, who has seen the slow, discouraging pace of efforts to fight viruses like Ebola and influenza, was stunned by the speed and success of Tuschl’s work. “In hepatitis C, it took decades to go from identifying the virus to having an effective drug,” he says. “Tom’s lab did it in under three years. That’s extraordinary.”

Fighting on multiple fronts
Tuschl’s strategy aimed squarely at SARS-CoV-2’s internal machinery. Meanwhile, another Rockefeller team was tackling the same enemy from a different angle: not by disrupting the virus’s tools, but by rearming the human immune system. For Hatziioannou and Bieniasz, the central question wasn’t how to break the virus—it was how to make our bodies better at recognizing and neutralizing it, even as it changed.
Their lab focused on a simple idea: Rather than generating a single, specialized antibody, what if they could coax the immune system into simultaneously producing a diverse arsenal of antibodies, each targeting a different part of the viral spike protein? This protein, which allows the virus to enter cells and initiate infection, was the main target of prophylactic vaccines and therapeutic antibodies during the pandemic. Traditionally, antibody therapies have relied on monoclonal antibodies—laboratory-produced molecules that precisely target one single vulnerable spot on a virus. This approach can be highly effective, but only until the virus mutates that one spot—something that SARS-CoV-2 proved more than capable of doing. A polyclonal response, by contrast, involves many antibodies at once, making it harder for the virus to mutate its way out of detection. If a virus changes one lock, the immune system still has plenty of keys.

“If the virus is mutating so rapidly, why not target something that’s more difficult to mutate? That, of course, is the host.” Hatziioannou
Initial experiments by Hatziioannou and Bieniasz, in close collaboration with Rockefeller colleagues in Michel Nussenweig’s lab, analyzed the blood of people who had recovered from COVID-19. People had many different antibodies to the virus, proving that there were multiple spots that could be recognized by antibodies.
Next, the researchers built pseudoviruses—lab-designed viruses that mimic SARS-CoV-2 but lack any ability to cause disease. The pseudoviruses allowed the team to study infection and immunity with greater speed and flexibility, free from the constraints of high-containment labs. “You take one piece of one virus and put it together with another piece of a different virus to make a virus that has useful properties,” Bieniasz explains.
Based on the antibody responses, and how those drove virus evolution, the researchers then designed protein immunogens to persuade immune cells to produce a variety of antibodies at once. If B cells—the immune cells that make antibodies—were exposed to two different versions of a virus positioned very close together, could they produce antibodies that recognized both?
“When that B cell receives a stronger signal from two related viral proteins simultaneously,” Bieniasz explains, “it’s more likely to generate antibodies that are capable of binding to multiple targets.”
This research reveals much about what will—and won’t—work to train the immune system to deploy a broader attack against a virus. After all, B cells are a central component of the human immune system, and learning how to make them better at recognizing divergent antigens could help fight off many types of viruses.
Closing a door
At the same time, Hatziioannou and Bieniasz also delved into the idea of making antibodies that bind to human cells, blocking the molecular door that the virus uses to gain entry.
“If the virus is mutating so rapidly, why not target something that’s more difficult to mutate?” says Hatziioannou. “That, of course, is the host.”
Many coronaviruses sneak into human cells through the same receptor, known as ACE2. But slamming shut ACE2 throughout the human body could have dire consequences because the protein also helps regulate blood pressure. In the past, this conflict would have been a death knell for a drug targeting ACE2. But in recent years, scientists have developed new tools to precisely control proteins’ functions, targeting one role without impacting their other jobs. As such, the number of antibody-based medicines has also been rapidly growing. Hatziioannou and Bieniasz saw a path forward.
The researchers isolated dozens of antibodies that mice generated and compared them against the human ACE2 protein. From there, they identified six antibodies that could successfully block viral infection without interfering with ACE2’s other biological activities. “It’s an incredibly clever approach to block the receptor, or to interfere with the ability of the virus to bind to the receptor,” says Rice.
The strategy worked even better than expected: The ACE2 antibodies prevented every tested strain of the COVID-19 virus, including tough-to-target Delta and Omicron variants, from entering human cells. It even prevented the first SARS virus, which caused an epidemic in the early 2000s. In mice, the antibodies protected against SARS-CoV-2 infection. “If this antibody had been developed early on, we think it would still be in use,” says Bieniasz.
The antibodies that Hatziioannou and Bieniasz created could be given prophylactically to people who are at high risk of serious disease, or as a treatment after infection. That kind of forward-looking strategy could be key if another coronavirus jumps from animals to humans, as SARS and SARS-CoV-2 once did. And it could potentially protect against another virus that binds to ACE2 that may still be waiting in the wings.
“If SARS-CoV-3 comes along tomorrow, then millions of people could benefit from this drug,” says Bieniasz.
Lessons from an invisible war
When the COVID-19 pandemic spurred labs across Rockefeller into action, they began with the basics: Know your enemy. Over the first months of exploration, they learned how SARS-CoV-2 infects, spreads, and kills. Then they began to learn the tricks that the virus uses to avoid immune recognition and treatment, which are shared across other types of viruses.
Ultimately, the best tactic to prevent the next pandemic from spinning out of control may be for scientists to have multiple tricks up their sleeves, ready and waiting.
“If we’ve learned one thing about developing antiviral drugs, it’s that you don’t want to go in with just one of them,” Rice adds.

That is why Rockefeller scientists haven’t stopped using SARS-CoV-2 as a test case—dismantling the machinery it relies on, enhancing the immune system’s memory, and locking the doors before the next virus can walk through. They’re playing the long game, looking not just for treatments that work today, but for approaches that can survive the viral evolution of tomorrow.
“Right now, there’s not a lot of incentive for a company to invest in a COVID-19 drug when there aren’t patients dying in the hospital,” says Tuschl. “So we could say, ‘Let’s just stop here.’ But then, when the next viral pandemic comes up, nobody wants to lock everybody up for the few years it takes to make a drug.”
Nothing about getting to this point has been easy. Each breakthrough—each antibody, each compound—is the result of years of molecular sleuthing and trial and error. But the momentum is real. And the payoff, if it comes, won’t just be another COVID drug. It will be a new way of fighting viruses—broad, durable, and ready before the next outbreak begins.
“We can’t predict what the next biggest viral threat will be,” says Rice. “But we can acquire the fundamental knowledge and prepare the tools that will help us fight it.”