Feature
Fat is beautiful, especially beige fat
Inside the energy-burning cells that just might hold the answer to some of our biggest health problems.
By David NoonanAs recently as nine years ago, fat was fat. It stored energy, caused disease, and made people feel bad about themselves. Drooping over the belts of millions of Americans, raising our blood pressure and decreasing our insulin sensitivity, fat was simply a burden, something to lose.
It turns out, however, that the decidedly unglamorous word “fat” belies a biological system as elegant, important, and challenging to understand as any in nature. Counterintuitively, fat, the reviled driver of the obesity epidemic, could potentially be harnessed in new treatments for a variety of obesity-related conditions, including type 2 diabetes, cardiovascular disease, and certain cancers.
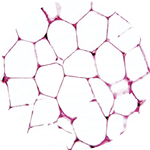
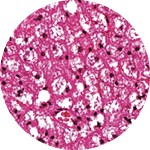
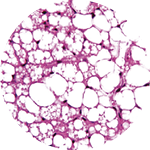
It’s a remarkable possibility that stems from the fact that not all fat is the same. Scientists have recently discovered that there are not one, not two, but three kinds of fat: white, brown, and beige. And while white fat has long been known to cause all sorts of trouble, brown and beige fat may actually promote good health.
In addition to just storing energy, brown and beige fat cells also consume it to produce body heat. For Paul Cohen, who heads the Laboratory of Molecular Metabolism, this makes fat itself a potential solution to an ongoing, ever-worsening health disaster.
when it comes to public health, few problems are bigger than obesity. According to the most recent data from the World Health Organization, more than 600 million adults worldwide are obese and another 1.3 billion are overweight. Findings from 195 countries, published in the New England Journal of Medicine in June, pegged the global prevalence of obesity at 12 percent for adults and five percent for children, more than twice the rates of 1980.
This crisis, which inspires Cohen’s research, is rooted in a deceptively simple equation. “If your energy intake chronically exceeds your energy expenditure,” he says, “that extra energy has to be stored somewhere. And it tends to be stored in fat.”
What if dropping the wrong kind of pounds will make us less metabolically fit?
Many researchers have focused on the food intake part of that equation, which has led to the development of several so-called diet drugs that act on the brain to curtail our desire to eat. But these therapies have been dogged by complications: “If you want to regulate appetite,” says Cohen, who is the Albert Resnick, M.D. Assistant Professor, “not only is it hard to get dramatic weight loss, it’s hard to do so without side effects, because the brain is so complex.”
He chooses instead to concentrate on energy expenditure. Cohen’s ultimate goal is to develop therapeutic methods for manipulating brown and especially beige fat to burn more energy, in order to protect people against obesity and its array of accompanying metabolic diseases.
before 2009, energy-burning brown fat was thought to exist only in certain small animals and in newborn humans, who need it to maintain their body temperature without shivering (brown and beige fat are both activated by exposure to cold). Its discovery that year in adults made headlines and launched a new era in the study of fat, which is technically known as adipose tissue. Then three years later, the discovery of beige fat in humans super-charged the growing field. Beige fat, found mixed in with brown fat in the neck and along the upper spine, has greater potential than brown fat, Cohen says, because it’s highly inducible—it changes quickly from a totally dormant state to a very active, energy-burning (or thermogenic) state. “From a therapeutic standpoint that’s exciting,” says Cohen, “because you could potentially toggle that switch back and forth.”

He began his obesity research working on both the intake and expenditure sides of the energy equation. He pursued his Ph.D. in the lab of Rockefeller’s Jeffrey M. Friedman, who in the 1990s discovered the appetite-controlling hormone leptin, earned his medical degree from Weill Cornell Medicine (he’s a cardiologist), and did his postdoc with Bruce Spiegelman at Harvard Medical School. He’s on his own now, leading a team of 12 grad students and postdocs, trying to unlock the inner workings of beige and brown fat, seeking to identify and understand the genes and proteins that govern their functions.
Cohen is particularly interested in a protein called PRDM16, which is known to be important both for the development of beige fat cells and for their ability to burn energy. Mice who lack the protein are unable to activate their beige fat and end up with many of the same features seen in human metabolic disorders, including insulin resistance, weight gain, inflammation, and, as suggested by Cohen’s recent and yet unpublished experiments, hypertension.
While many scientists in the field are trying to understand how PRDM16 functions, to Cohen the protein is more of a tool. For his experiments, he breeds mice that either lack PRDM16 or produce it at natural or elevated levels. He and his team then use these different animals to study how beige, brown, and even white fat cells behave under various conditions.
Their goal is to develop interventions that augment beige fat activity by manipulating PRDM16. Accomplishing this without producing significant side effects might be easier said than done, however, because PRDM16 is expressed in a wide variety of cell types, and plays an important role in crucial biological processes, including the differentiation of stem cells into mature cells. “For example,” says Cohen, “PRDM16 is involved in the development of cells in the blood lineage, so there is the very real concern that if you activated it in those cell types you could get leukemia.”
A less risky proposition would be to figure out a way to make a drug that would target the protein only in fat cells. It’s an approach that could ultimately lead to the holy grail of fat research—new drugs to treat obesity and its related diseases.
“It’s conceivable,” Cohen says, “that if we identified a kinase that regulated PRDM16 in fat cells and found a way to modulate it, we might ramp up the metabolic activity of beige fat.”
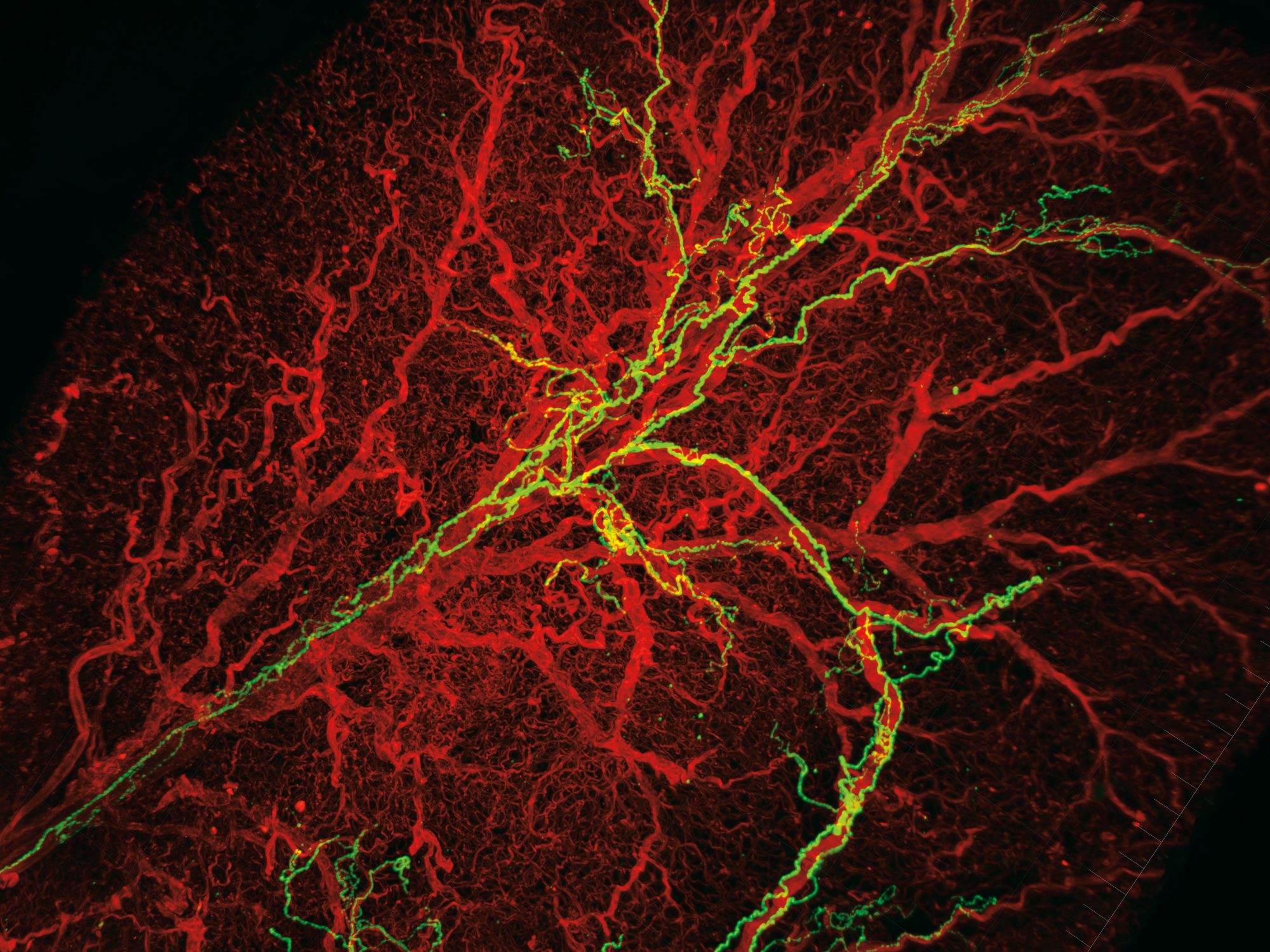
the biology of fat is intricate in itself, yet Cohen says that to fully understand how adipose tissue functions, one needs to consider its interactions with the nervous system, which plays an important role in letting fat cells know how much energy to store and how much to expend. Jingyi Chi, a grad student in the lab, set out to examine the tissue-level organization of the fat, including its innervating nerve cells. She hit a roadblock almost immediately. Scientists who study fat were missing quality imaging—few people had developed tools to look at adipose tissue in detail. So she borrowed and modified a revolutionary three-dimensional tissue imaging system known as iDISCO, developed in another Rockefeller lab to study the brain, that makes it possible to “clear” the tissue of lipids and reveal the structures underneath.
“When people think about adipose tissue, it’s just a lump of fat,” Chi says. But her experiments exposed a lot more: a stunning biological architecture within the lump, including long, luminous green neurons and a glowing red web of blood vessels. And beyond being an aesthetic revelation, the results were scientifically stunning.
For instance, the images revealed that nerve cells form projections called neurites—which Chi says are necessary for the activation of the beige fat—to a different extent depending on the presence or absence of PRDM16 in the fat cell. “This suggests some dialogue where, if you have PRDM16 in a fat cell, it somehow guides the neurites to the site,” Cohen adds. “And if you take it away you don’t have those neurites.” His team is now working to identify the molecular components of this dialogue, insights into which could offer yet another way to manipulate beige fat and fight obesity.

like every good scientist, Cohen is careful not to oversell his research, but his enthusiasm for the therapeutic potential of beige fat is unmistakable. He is especially interested in it as a possible weapon against diabetes. More than 29 million people in the U.S., nine percent of the population, have the disease, one of the grim hallmarks of obesity. One of Cohen’s ideas for a possible treatment grew out of the observation that mice that lack PRDM16 become insulin resistant at the level of the liver, a condition that can lead to type 2 diabetes. This was a strong and consistent finding, and Cohen and his lab members wondered if there might be some kind of molecular signal from beige fat to the liver that regulates insulin sensitivity—a signal that is disrupted in the absence of beige fat activity.
Sean O’Connor, another grad student in the lab, confirmed their suspicion by growing liver cells in a petri dish and bathing them in media recycled from other dishes used to grow fat cells. The results from his deceptively simple experiment showed that molecules secreted by white fat cells increased glucose production and decreased insulin sensitivity, which is bad, while those produced by beige fat cells had the opposite effect, improving insulin sensitivity and reducing glucose levels, which is good.
The findings are preliminary, Cohen notes, but “this suggests that there is some direct interaction between the fat and the liver that’s important. What we hope is that this will lead to the discovery of a new beige fat-derived factor that regulates hepatic insulin sensitivity.”
encouraged by these findings, Cohen is turning his attention to other obesity-related diseases—for example, he is exploring the role of beige fat in regulating blood pressure and slowing the growth of breast tumors. But perhaps the most surprising takeaway from his work is that, when it comes to health, how much you weigh is less important than the type of fat you have. Even where you carry that fat matters: visceral fat, found in the belly, is associated with metabolic disease, while subcutaneous fat, on the hips and elsewhere, likely contains some beige fat and may be considered healthy.
As a result, some people who are far heavier than their recommended weight, including a former female patient of Cohen’s who weighs well over 300 pounds, don’t have diabetes, high blood pressure, or any of the other disorders that plague most obese people. They are what he calls MHOs, the metabolically healthy obese, and their extra pounds tend to be distributed around their hips rather than in their bellies. Meanwhile, other people who are at or below what’s considered a healthy weight nevertheless suffer from obesity-related diseases. India, for example, is experiencing a huge increase in the incidence of type 2 diabetes, Cohen says, and there the typical patient “might weigh less than 150 pounds, but have a slight paunch.” That paunch, made up of visceral fat, appears to be enough to cause serious problems.
All of which turns our national obsession with weight loss on its head. Losing the gut is always a good idea—the less visceral fat the better. But what if dropping the wrong kind of pounds—from the hips, for example—could make us less metabolically fit, if we lose precious beige fat cells in the process? The message from Cohen is that having an overweight, pear-shaped body isn’t all that bad. It may even be healthy.
“Beige fat cells may not promote weight loss so much,” Cohen says, “but they promote metabolic health, and that’s fine. At the end of the day, for our vanity, we might like to get back to the weight we had when we finished high school, but our health is much more important than what the scale says.”