Feature
In an embryo’s second week, a surprise
Rockefeller biologists opened a window into the mysterious period when a human embryo first attaches to its mother’s uterus—and what they saw amazed them.
By W. Wayt GibbsWe all begin as a tiny, hollow ball of about 150 cells, rolling around in the uterus. Whether conception happens in a test tube or the old-fashioned way, the embryonic ball, called a blastocyst, has to accomplish a crucial early task to earn a shot at life: It must stop rolling and attach itself to the walls of the uterus, where it will be supplied with food and oxygen for the next 40 weeks.
Many embryos fail this test. For reasons that remain mysterious—scientists have never witnessed the magical moment of implantation in humans—a substantial fraction of embryos either don’t stick at all or take more than nine days to implant, with each additional day reducing their chances of survival.

“We know absolutely nothing about what goes wrong,” says Ali H. Brivanlou, Robert and Harriet Heilbrunn Professor and head of the Laboratory of Stem Cell Biology and Molecular Embryology at Rockefeller. “Embryos’ failure to attach is the biggest problem that fertility doctors see when they perform in vitro fertilization.”
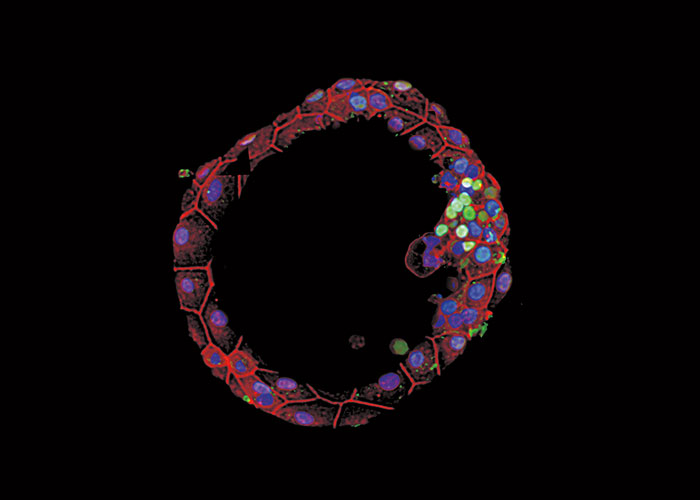
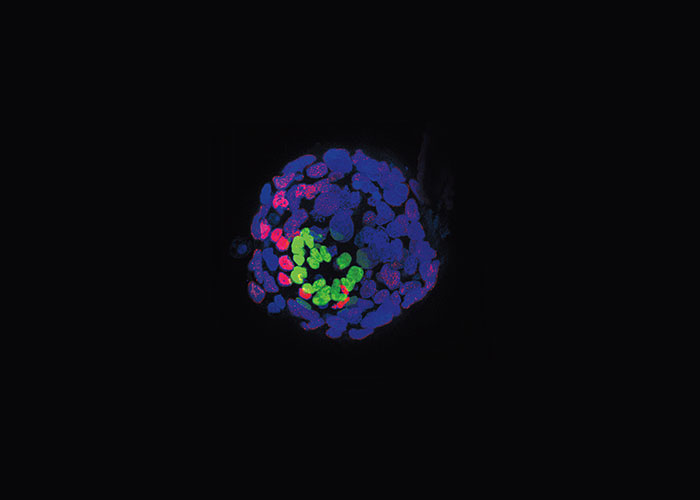
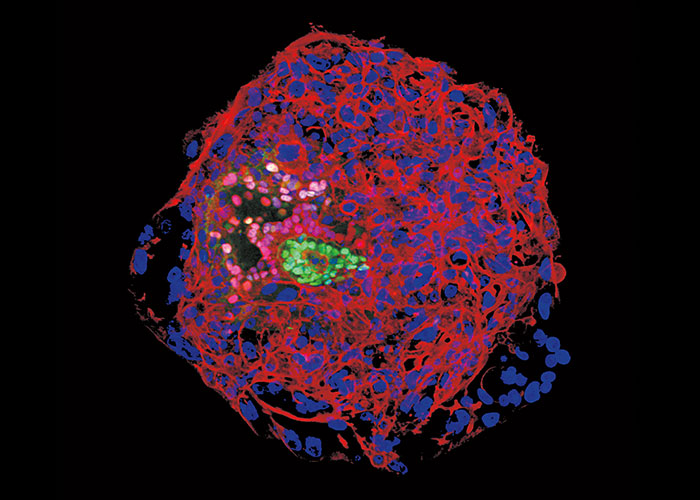
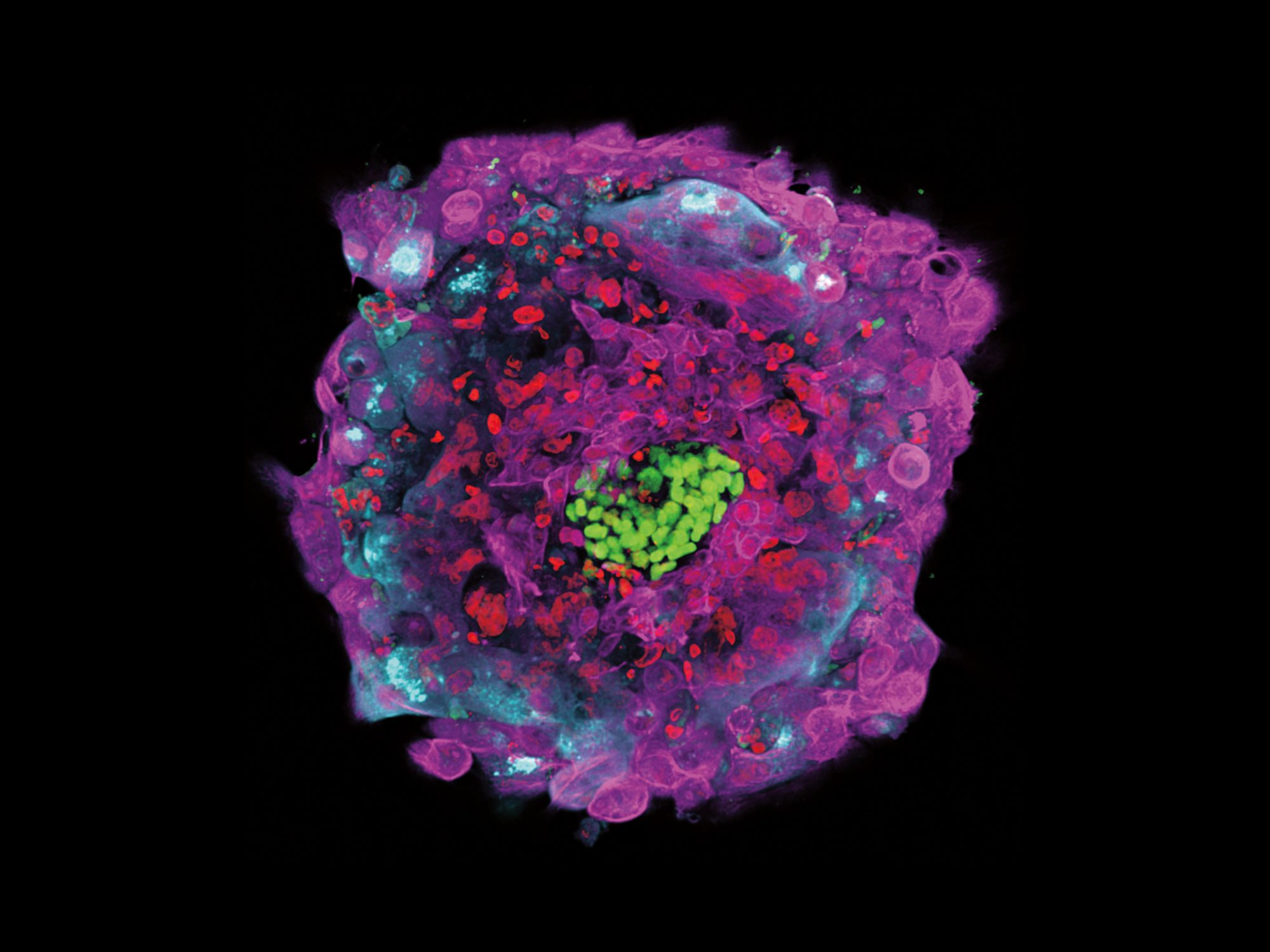
But Brivanlou’s team recently pulled back the curtain on this momentous event in human development. By applying the Rockefeller group’s special expertise in working with embryos of many kinds, from frogs to humans, Brivanlou and research associates Alessia Deglincerti and Gist Croft, and research specialist Lauren Pietila, were able to accomplish what no one had done before. They established a reliable technique to sustain human embryos in petri dishes all the way through the second week after fertilization, and to record in detail how the blastocysts attach to a plastic scaffold (in lieu of a womb), flatten into discs, and begin to develop under the control of an internal program.
It’s a breakthrough that gives biologists a remarkable opportunity to fill in many of the missing pieces in the puzzle of the earliest stages of human life. That more complete picture could in turn lead to concrete medical advances, starting with insights into why so many pregnancies fail before implantation or within the first two months afterward.
“If someone had told me when I was in grad school that I would ever be able to witness this stage of human development, I would have thought they were crazy,” Brivanlou says.
Consider, for instance, one of the most fundamental questions in early human development: whether human embryos are able to develop properly without being connected to the maternal uterus, which supplies vital biochemical signals. The new research shows that all of the information necessary to control attachment and the next few steps of development resides in the embryo itself. While hormones and other signals from the mother certainly become crucial at some point in development, that point occurs later than biologists had assumed.
“The fact that communication between the mother’s tissues and the embryo isn’t necessary for attachment to happen was a big surprise to the field,” Brivanlou says.
The research has other important implications. By labeling the embryos with molecular markers that can be lit up with lasers, and monitoring the order in which genes are turned on and off during this crucial period of development, scientists are now able to detect when things go wrong on a molecular level. “Genetic errors and other defects can start to pile up. Even if the embryo survives, such errors could potentially cause disease or complications later in life,” Brivanlou says. If so, it would be good to know how to detect, prevent, or correct the defects.
The researchers are also excited about the emerging potential of using embryonic stem cells to model diseases of many kinds in the lab. A subset of those 150 cells in the blastocysts, embryonic stem cells have the ability to regrow brain tissue, to reconstitute the immune system, or to differentiate into any of the body’s many cell types. But scientists need more knowledge, specifically from human cells, for such approaches to become effective, says Croft. “We have to understand where embryonic stem cells are coming from, and what decisions they’ve made or are about to make,” he says. “Only then will we be able to control their ability to become the cell types that are useful for drug screening and transplantation.”
Currently, the use of cells derived from human embryos is limited by both ethical and practical constraints. One big problem, Brivanlou notes, is that when researchers grow new tissue from these stem cells, success is largely a matter of trial and error because we don’t understand well enough how the processes of differentiation work in the body—how a stem cell naturally gives rise to bone cells, for example. “Our new method may let us observe, for the first time, how the human body does this itself,” he says. While others work on the applications, Brivanlou and his team are focusing on further improving the technique so that it will reproduce more faithfully how embryos develop during normal pregnancy.
Deglincerti adapted the new approach from a similar method used to study fetal development in mice, established by a colleague at the University of Cambridge, Magdalena Zernicka-Goetz. “We carefully manipulate the embryos in a dish, and use chemicals to peel off a thin envelope that surrounds the embryo at this early stage,” Deglincerti says. “We then bathe the naked blastocyst in hormones and growth factors.” With this combination, the group was able to grow the embryos through attachment and up to the 14th day of development.
They stopped there. According to international ethical guidelines for conducting experiments on human embryos, 14 days is the cutoff. But those ethical guidelines were set up many years ago, when the 14-day limit seemed well out of reach. Now it may be time to reevaluate them, Brivanlou says, since his group has shown that it’s technically feasible to culture embryos to the 14-day limit, and possibly beyond.
“Today we would not want to attempt going past day 14, even if the regulations allowed it,” he says, “because anatomical abnormalities would start to appear in the embryos.” However, scientists may soon find a way to keep development proceeding normally for longer than two weeks. And at that point, technology will no longer be the limiting factor, yet the ethical concerns will remain. “We need to have a debate throughout all society about whether in certain cases it could be acceptable to allow embryos to develop further in vitro in order to gain more insight into human development,” Brivanlou says.

While there is no predicting what discoveries could follow from allowing expanded research, the very first glimpses through this new window between days 9 and 14 turned up something quite unexpected. While sifting through the mass of results from the many tests they had run on individual cells inside the embryos, the team stumbled upon a new kind of cell, which they named yolk-sac trophectoderm cells.
“Nobody has seen this cell type in any animal before,” Brivanlou says. “We reproduced our experiments 10 times to convince ourselves. It is there, and it is specific to humans. But we have no clue what these cells are doing. Are they like the tail and gills—structures that appear in the womb but then vanish before birth? Or do they give rise to something that stays with us throughout life?”
Questions like these often pop up when scientists crack open a black box and peek inside. It makes you wonder: What else is in there?