Feature
Walking the line
Inflammation is both our body’s best natural defense and the underlying cause of many serious, chronic illnesses. How do you treat a dangerous condition that’s also a fundamental biological process?
By Alexander GelfandW
hen Rockefeller immunologist Jeffrey V. Ravetch asks his students to describe the function of the system he’s studied for the better part of four decades, he typically gets a variety of answers. But his own is always the same. “Its primary function is not to kill you,” Ravetch says. “Because given the chance your immune system will wipe you out.”
At first, Ravetch says, students may need a moment to take that in: It can feel counterintuitive to reframe the body’s tools for warding off infections and recovering from injuries as simultaneously life-threatening. But that duality is exactly the point.
Ravetch, the Theresa and Eugene M. Lang Professor, has spent his career probing the mercurial properties of antibodies and demonstrating how the subtlest molecular tweaks to this or that receptor can result in dangerously different outcomes. Antibodies are famously the means by which we neutralize an invading virus or shut down a burgeoning cancer. Yet they can also be pathogenic in and of themselves, spontaneously triggering a runaway inflammatory process that scientists now know can lead to enormous harm.
“How,” Ravetch asks, “does one molecule accomplish all these diverse functions?”
The answer lies at the heart of one of the biggest questions currently vexing medicine: how to rein in uncontrolled inflammation.
In recent years, our body’s natural defense system has been implicated in a growing list of serious and chronic conditions, from food allergies and colorectal cancer to obesity and autoimmune disease. Driving conditions from asthma to lupus and increasing the risk of everything from Alzheimer’s to heart disease, inflammation has even been linked to the degenerative effects of aging.
As awareness of these health impacts spreads, an entire anti-inflammatory industry has sprung up, with advocates touting cold plunges, special diets, and other lifestyle changes meant to calm the system. Some of these trendy life hacks may turn out to be truly beneficial; the science is still out. But without understanding the basic biological mechanisms that drive inflammation and its effects, trying to manage the phenomenon by jumping into a tub of ice water or eating a bucket of blueberries is like throwing darts at a board. And none of the trends addresses the fundamental quandary of how we should treat a dangerous condition that is also an essential biological process.
This is where researchers like Ravetch and Rockefeller colleague Elaine Fuchs, who focuses on the biology of skin stem cells, come in. Their labs are a part of a larger effort to identify what separates the restorative effects of inflammation from its pathological ones, and how the line between the two can become blurred or even erased entirely. What they are learning is just how complex inflammation truly is, and how many different systems intersect to produce and regulate it. All of which, they say, boils down to one central problem:
“A little bit of inflammation is a good thing,” says Fuchs. “But how do you control it?”
Doctors still learn the signs and symptoms of inflammation by reciting a Latin phrase invented by the Roman scholar Celsus 2,000 years ago: calor, dolor, rubor, tumor—heat, pain, redness, swelling. (The term itself comes from inflammare, to set on fire.) By the 18th century, scientists had begun to relate those overt characteristics to the microscopic processes (increased blood flow, fluid buildup, a rush of white blood cells) that the body relies upon to defend and repair itself. But while it was understood that inflammation represented one of our best tools for survival, the question of how it fought infection, killed dysfunctional cells, and repaired damaged tissues remained largely unanswered.
Centuries later scientists are still teasing out inflammation’s causes and effects. But one thing has become increasingly clear: Its dark side emerges when protective mechanisms run amok, failing to shut down when they should (e.g., chronic inflammation) or attacking healthy tissues when they shouldn’t (e.g., autoimmune disease).
Nearly 25 years ago, scientists came upon a conundrum that neatly encapsulated this fickle quality. As antibody therapies first entered clinical use, doctors began to notice something odd: Giving people with compromised immune systems infusions of these drugs helped them mount the inflammatory responses necessary to fight infection. But administering larger doses of the same antibodies suppressed inflammation in other patients with autoimmune disease.
Nearly 25 years ago, scientists came upon a conundrum that neatly encapsulated the fickle quality of antibodies.
Ravetch, who heads the Leonard Wagner Laboratory of Molecular Genetics and Immunology, was uniquely equipped to explain this apparent paradox. Long fascinated by how antibodies trigger inflammation to eliminate threats and suppress it once they’ve passed, he has focused on a component of their structure known as the Fc region, or “the Fc.” Most immune cells have receptors that either activate or inhibit inflammation by binding to the Fc, and Ravetch has both explored and exploited the region’s pro-inflammatory and anti-inflammatory capabilities in many contexts, and for many purposes.
In this case, Ravetch discovered that the seemingly contradictory effects of low versus high doses of antibodies were in fact caused by chemical changes to the Fc. Some changes ratcheted up inflammation, while others tamped it down. But since the anti-inflammatory changes were rarer, their effects become pronounced only at high dosages. Ravetch and his colleagues subsequently figured out how to engineer these anti-inflammatory effects to create more effective autoimmune therapies, the first of which is now entering clinical trials. Standard antibody therapeutics, which are used to treat a variety of serious inflammatory autoimmune disorders affecting the heart, blood vessels, and nervous system, must be made from the pooled blood of thousands of donors and given in large volumes to patients over the course of several days, making these treatments hard to come by and difficult to administer. Because the drug developed from Ravetch’s lab is 10 times more potent than current treatments for rare but potentially disabling conditions like Guillain-Barré syndrome and Kawasaki disease, however, it could solve both problems.
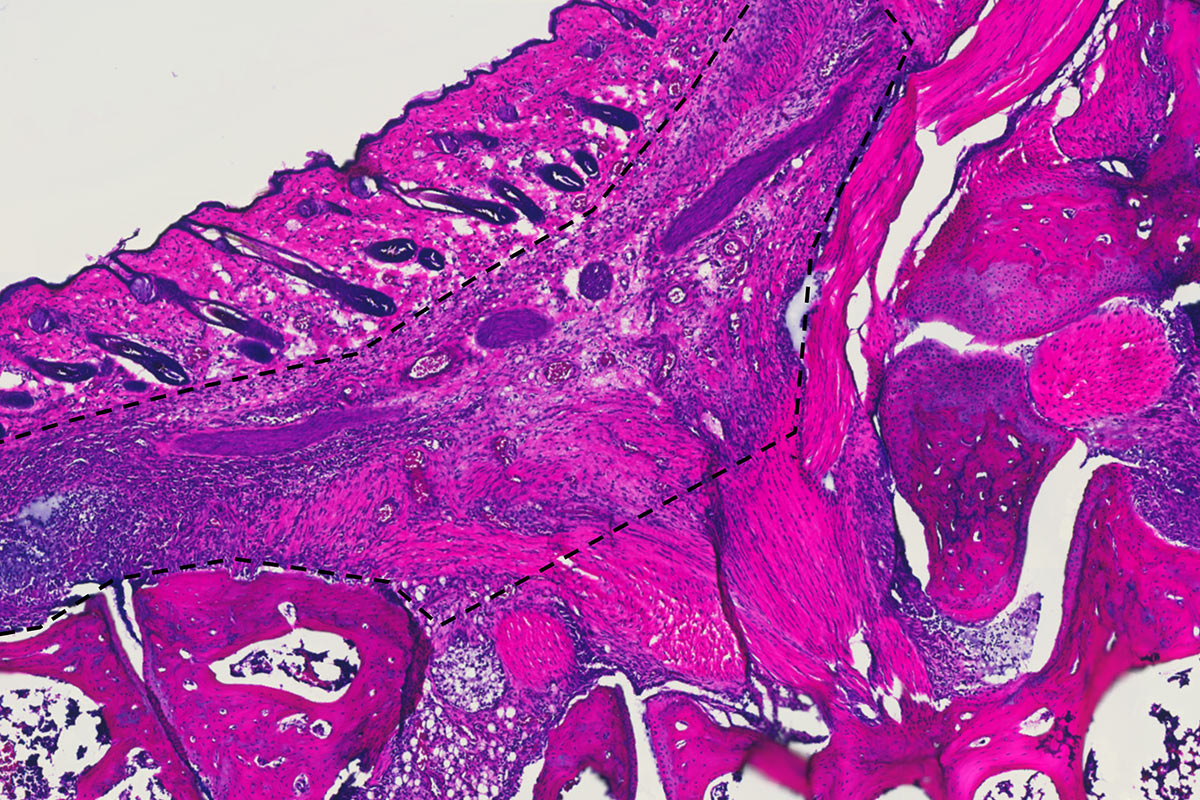
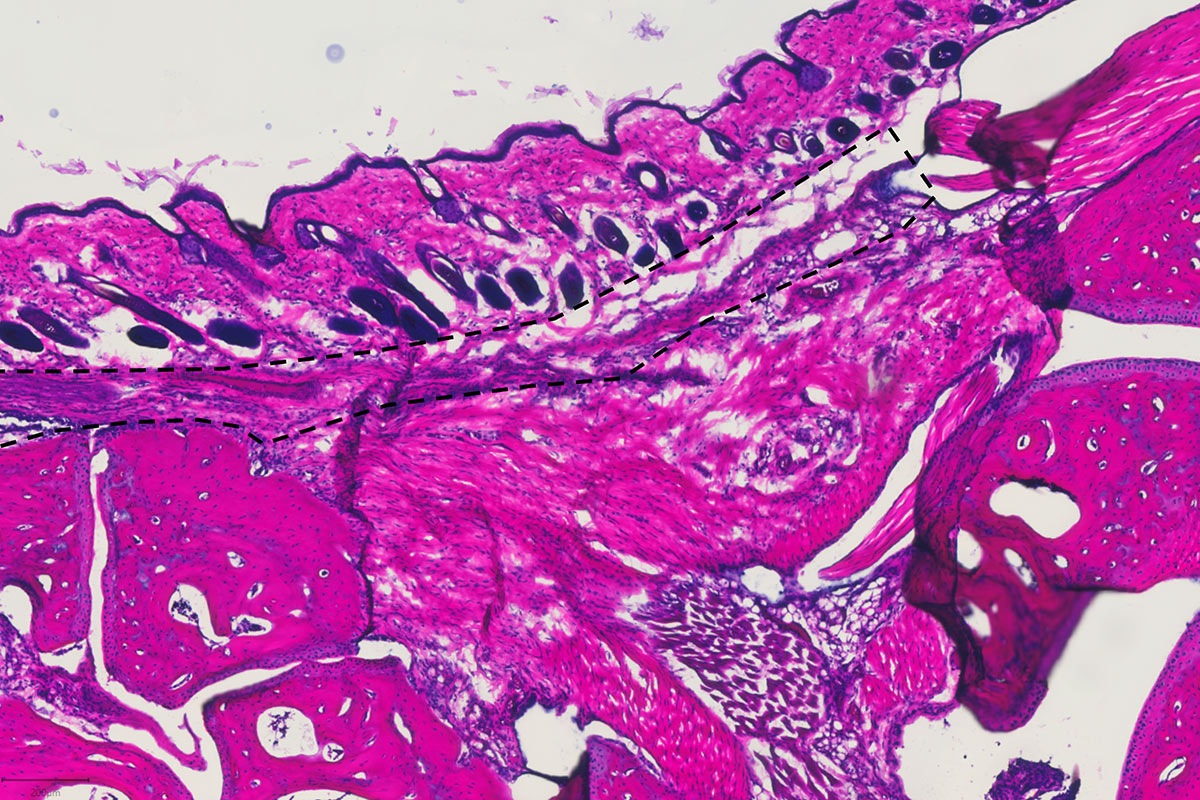
Ravetch’s team has also tinkered with the Fc to increase the efficacy of therapeutic antibodies used to treat cancers of the blood, breast, and colon. The use of such drugs, known as immunotherapy, has revolutionized cancer care in recent years; augmenting their inflammatory power has made them even more effective. “About a dozen antibodies have been re-engineered based on this understanding that Fc modifications can enhance their pro-inflammatory properties,” he says.
Ravetch is also leveraging the Fc to identify people who are at high risk of experiencing severe infectious disease, the most harmful effects of which are often caused by rampant inflammation. Dengue, or “break-bone” fever, is a good example of this. In most people, the viral illness is painful but not life-threatening. In some, however, the body’s inflammatory response is so extreme it can prove deadly. Why? The difference, Ravetch discovered, boils down to a pro-inflammatory modification they carry in this region.
This finding allowed Ravetch to develop an antibody that can screen for patients who are at risk of developing severe dengue—and may even be able to prevent it. Another illness whose worst effects are caused by runaway inflammation? COVID-19. Ravetch hopes to employ the same method to screen for—and develop strategies to protect—high-risk patients.
Our skin bears the brunt of what the world throws at us, from scratches and scrapes to allergens and ultraviolet radiation. Elaine Fuchs had long been fascinated by the biology of the organ’s underlying stem cells, and by how they generate new tissue and repair wounds. Her lab has produced a long string of insights at the intersection of genes, aging, and cancer.
About a decade ago, however, Fuchs’s curiosity about stem cells led her in an unexpected direction. It started with another widely observed phenomenon that had never been adequately explained: Why is it that inflammation will often strike a particular location on the skin (an angry red spot on the arm, a scaly patch on the upper thigh) in response to a specific stimulus like a wound or allergy; resolve completely once the stimulus disappears; then reappear in exactly the same location years later in response to an entirely different stimulus? And why is the second response often stronger than the first?
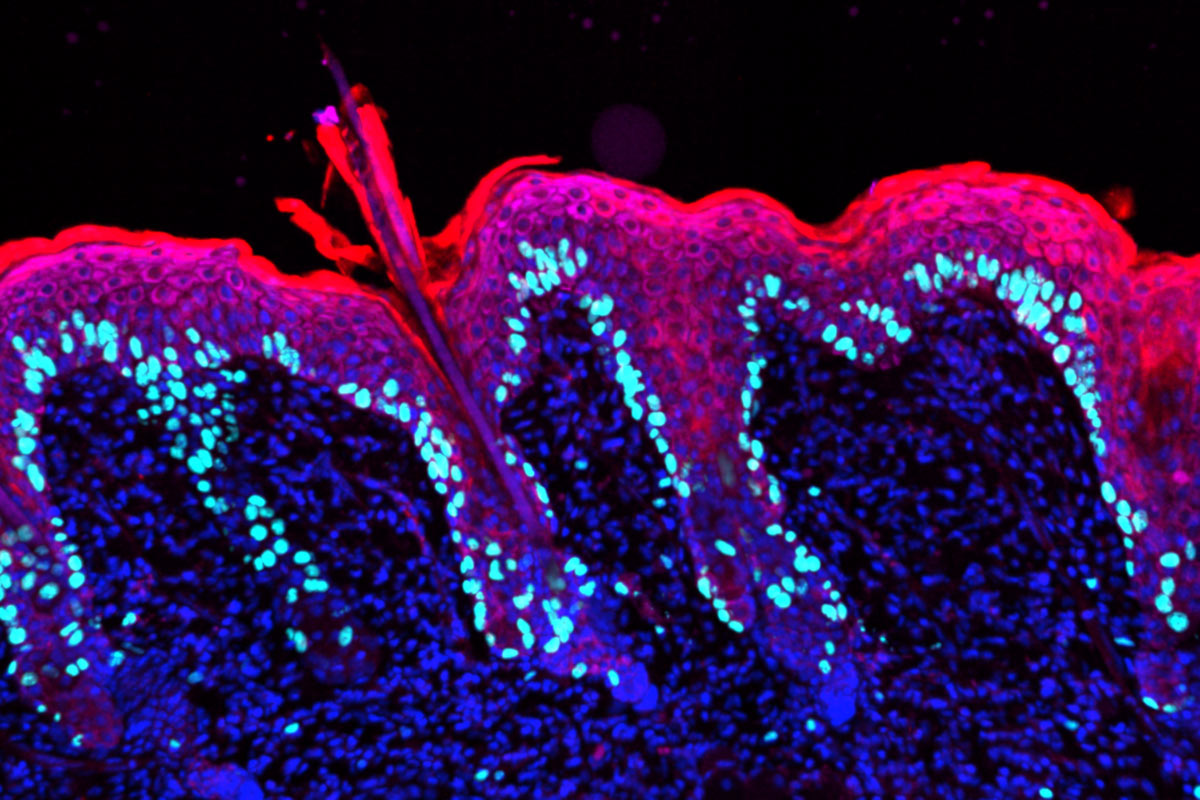

To investigate, Fuchs, who is the Rebecca C. Lancefield Professor and heads the Robin Chemers Neustein Laboratory of Mammalian Cell Biology and Development, met with her team and developed an experiment that involved applying a chemical irritant to one side of a mouse’s back. After the resulting inflammation died down, a mild wound was administered to the same spot, as well as to the side that had not been irritated. Remarkably, the spot that had previously been inflamed healed faster than the one that had not—even though the new irritant was different from the original source of inflammation.
Intrigued, Fuchs set out to identify which biological actors were hastening the healing process—and discovered a mechanism that changed how scientists think about inflammation in general.
Many researchers had believed the likeliest culprits behind this inflammatory memory were immune cells, which have long been known to store details of past encounters with pathogens—a phenomenon also known as trained immunity.
Much to the surprise of the scientific community, Fuchs found that long-lived epidermal stem cells sat in the driver’s seat, retaining and recalling memories of inflammatory incidents without the help of immune cells. During the first inflammatory episode, inflammation response genes turned on, allowing the stem cells to enter repair mode. But while most shut back down after the initial inflammation had resolved, some DNA “inflammation sensors” associated with these response genes remained active for up to six months in mice—or approximately five to six years in human terms. The next time these educated stem cells faced an inflammatory stimulus, they were ready to respond more quickly and robustly, even when the new stimulus differed from the old one.
This dynamic helps explain the skin’s ability to quickly jump-start a reaction to stressors it has never experienced before. It also helps explain why local sensitivity to an inflammatory response can linger long after the stimulus that provoked it has vanished. “While lifesaving for wound healing, this can lead to chronic inflammatory disorders,” Fuchs explains. As a result of Fuchs’s work, researchers are now pursuing the role that this previously unknown mechanism may play in other tissues where chronic inflammatory conditions can occur.
Fuchs herself is looking at how she can leverage her discovery to address a whole host of disorders, from skin conditions such as psoriasis and atopic dermatitis to inflammatory bowel disease.
Her team is also exploring the relationship between inflammation and cancer—in particular, squamous cell carcinomas, which affect not only skin but also the head, neck, lungs, esophagus, and cervix, making them one of the most common and life-threatening forms of cancer worldwide.
The skin is remarkably adept at clearing cancer cells, in large part thanks to interactions between skin stem cells and the immune system. Yet once a tumor has formed, inflammation can actually feed the malignancy by promoting cell proliferation, a process that is largely driven by disordered communication between cancerous stem cells and their environment. Determining how and why that switch occurs could lead to novel therapies not just for skin cancer but also for tumors that form in other parts of the body that aren’t nearly as adept at dealing with the disease.
“Once we figure out why skin stem cells are so good at responding to insult and communicating with the immune system, we hope to be able to control the response in chronic inflammatory disorders—and also teach other parts of the body how to better respond in potentially cancerous situations,” Fuchs says.
In scientist Daniel Mucida’s research, balance is everything. Mucida studies intestinal tissue, which has become the subject of intense scrutiny in recent years.
For one thing, the gut is extraordinarily complex, comprising multiple layers of tissue and more neurons than are found anywhere outside of the brain, to which it is directly connected.
For another, it plays an essential role in maintaining our overall health. Indeed, if the skin is the primary barrier between our bodies and the outside world, the gut is the principal portal between the two: a place where everything we swallow—food and drink, toxins and parasites—eventually winds up.
This constant influx of foreign material, coupled with the presence of our own gut microbiota—the collection of (mostly) helpful microbes that have colonized it—means that the gut is in a constant state of inflammation: The epithelial tissue that lines the intestines, and the immune cells that patrol it, are forever sorting friend from foe, generating protective responses against dangerous invaders while tolerating everything else. When that system goes out of whack, however, uncontrolled inflammation can lead to painful and potentially deadly conditions like colitis, food allergies, and cancer.
Mucida, who heads the Laboratory of Mucosal Immunology, is working with his team to understand how the gut strikes this exquisite balance between tolerance and resistance at a cellular and molecular level, and what happens when that balance breaks down.
Mucida and his colleagues are investigating, among other things, T cells, a diverse group of immune cells that recognize a huge range of antigens, kill infected and cancerous cells, and coordinate the activities of other immune cells. Under normal conditions, T cells are exceptionally good at distinguishing harmless residents and interlopers from dangerous ones, and they play an important role in detecting nutrients, sensing infections, and preventing allergic reactions.

“But they can also go crazy and cause disease,” says Angelina Bilate, a research associate in the Mucida lab. One such example is celiac disease, an autoimmune disorder in which T cells react to gluten by attacking the small intestine.
By studying the antigens that T cells recognize and the receptors they use to do so, Bilate aims to describe the whole spectrum of inflammatory responses that a typical individual’s gut can mount. That, in turn, should allow her to determine how that spectrum changes with illness, as otherwise tightly regulated responses go unchecked.
In a similar vein, graduate student Marwa Saad is exploring how immune cells monitor the gut, and how they communicate with epithelial cells to coordinate that surveillance. Most recently, she has been using cancer as a model to understand how the immune system perceives and responds to insults to the gut, and how communication between immune and epithelial cells can be disrupted.
Under healthy circumstances, immune cells zip from epithelial cell to epithelial cell in a pattern called “flossing.” With each pass the immune cells scan the epithelial cells and receive information about pathogens in the gut. When tumors form, however, the immune cells can no longer “see” the epithelial cells properly, and communication breaks down. Figuring out how that happens—and how to restore proper perception and communication—could lead to new therapies for colorectal cancer and other illnesses.
Psoriasis is an inflammatory skin disease that affects between two and three percent of the global population. It most often presents as itchy, scaly lesions called plaques that are both uncomfortable and unsightly. But it can also prompt a massive inflammatory response, sending patients rushing to the ICU. In very rare cases, it can kill a person.
When dermatologist James G. Krueger began practicing medicine in the 1980s, the drugs used to treat its most dangerous forms were blunt instruments that suppressed the entire immune system—the medical equivalent of throwing the baby out with the bathwater.
Krueger was intent on finding safer and more effective therapies for the disease. In the 1990s, he and his colleagues conducted experiments that indicated psoriasis was most likely caused by abnormal activity among T cells. In the case of psoriasis, they appeared to be generating inflammation in response not to some foreign pathogen but to a substance that the skin itself generated, making psoriasis an autoimmune disease as well as an inflammatory one.
Unfortunately, there were no trustworthy animal models for psoriasis that Krueger could use to identify specific inflammatory pathways and test potential treatments. He realized, however, that drug companies had already developed a number of molecules that targeted various pathways by blocking inflammatory signaling molecules called cytokines. So, he and his team came up with a creative approach to trace back the root cause of the disease.
They began a series of meticulously conducted small clinical trials at The Rockefeller University Hospital, where Krueger is now senior attending physician, giving psoriasis patients existing drugs that blocked different cytokines and carefully analyzing the results back in the lab. This allowed them to simultaneously identify the pathways underlying the disease and effective treatments for it.
It was the scientific equivalent of finding a needle in a haystack—and then deducing how the needle had found its way into the haystack in the first place.
Krueger’s small trials eventually led to larger ones, which in turn led to FDA approval for a whole new class of psoriasis drugs that are safe and effective in approximately 90 percent of patients. As a result, says Krueger, who heads the Laboratory of Investigative Dermatology, psoriasis is now “the best-treated inflammatory or autoimmune disease, period.”
What’s more, psoriasis is only one of several T-cell-mediated inflammatory skin conditions that together affect 10 to 15 percent of adults worldwide—and Krueger’s bed-to-bench-and-back approach can be applied to all of them. Atopic dermatitis, for example, affects as many as 20 percent of all infants; as was once the case with psoriasis, the only treatments for its most severe forms were themselves potentially dangerous. In recent years, however, researchers have identified safer and more effective therapies by adopting Krueger’s method of probing the underlying mechanisms of the disease with cytokine-blocking drugs. And Krueger himself, the D. Martin Carter Professor in Clinical Investigation, is currently working to identify new, pathway-targeted treatments for an excruciatingly painful inflammatory skin condition called hidradenitis suppurativa.
But his work has implications far beyond the skin. A paper Krueger co-authored in 2021 showed that patients with psoriasis were 50 percent more likely to develop cardiovascular disease—a finding that indicates inflammatory skin diseases can be signifiers of other serious disorders, and not just ones that are commonly thought of as being inflammatory in nature, like arthritis and asthma. (Research by other scientists suggests that psoriasis patients are also more likely to develop dementia.) Sometimes, Krueger says, such comorbidities are due to shared genetic factors and common disease pathways. But they are also likely driven by something else—namely, the passage of cytokines from the skin into the bloodstream. “Every tissue in the body starts to be bathed in an inflammatory cytokine mix,” he says.
The resulting constellation of inflammation-related health problems can reduce the lifespan of a psoriasis patient by five to 10 years—years the new drugs Krueger helped develop now promise to restore.
Such discoveries illustrate why it’s hard to overstate how much harm inflammation can do when it gets out of hand, and how much good can be done by reining it in. And they represent just the beginning of what Rockefeller scientists hope to achieve.
Ravetch, for instance, recently showed that by modifying the Fc in an antibody-based cancer drug that had shown promise in mice but failed in humans, he could generate cancer-killing effects that were both powerful and long-lasting: Patients who received the Fc-enhanced drug in a clinical trial at The Rockefeller University Hospital not only saw their skin and metastatic breast cancer tumors melt away but also remained cancer-free for months afterward. Trials are now underway at other institutions to test this potentially revolutionary treatment on brain, bladder, and prostate cancer.
Fuchs, meanwhile, is exploring the role of inflammatory memory in chronic inflammation. She and her colleagues have already identified hundreds of skin stem cell genes that act as inflammation sensors after undergoing an inflammatory experience, just waiting for some fresh insult to occur so that they can leap into action. Under normal circumstances, once the inflammatory stimulus has subsided, everything goes back to normal. Now, however, she and her team are designing experiments to reveal what happens when that cycle of inflammation and relief repeats over and over again in an endless loop, battering the skin without mercy. “When you keep doing it, what will you get?” she asks. “Can the skin figure out what to do?”
On the gastrointestinal front, Mucida is investigating the inflammatory relationship between the immune system and the nervous system—a particularly promising line of inquiry for gut researchers, since the organ is loaded with neurons that communicate directly with the brain and spinal cord. For instance, Albana Kodra, a postdoctoral associate in his lab, is looking at allergic reactions in mice to see if the nervous system can act as a reservoir of inflammatory memory. If so, identifying the biological mechanisms at work could lead to new treatments for food allergies in humans.
And Krueger, who has already helped deliver life-changing drugs to millions of patients, is now turning his attention to Lyme disease. While most people who acquire this tick-borne infection suffer nothing more than a distinctive skin rash and flu-like symptoms, a small subset goes on to develop chronic inflammatory conditions characterized by heart problems, nerve damage, and brain fog. A collaborative team of clinicians and scientists at other institutions has asked Krueger to help explain this phenomenon, and he hopes to discover why the inflammation associated with Lyme disease sometimes spreads beyond the skin—and whether such systemic inflammation can be treated with the same drugs that are used to combat inflammatory skin diseases.
Projects like these promise to solve some of the enduring mysteries of inflammation: Precisely which cellular and molecular actors control it, and what role does memory play? How does inflammation both contribute to cancer and protect us from it? And why does the immune system’s ability to mount an inflammatory response against the right targets, or shut that response down at the right time, sometimes fail to such devastating effect?
Answering these questions will help scientists further understand and harness the power of inflammation—a power that can sometimes be tamed, but never overestimated.
“The body’s defenses,” Ravetch says, “must be respected at all times.”